
TOPIC 6: AIR COMBUSTION, RUSTING AND FIRE FIGHTING.
Composition of air
Air is a mixture of different gases. The gases that make up the air include nitrogen, oxygen, carbon dioxide, noble gases (argon, helium, neon, krypton and xenon) and a little water vapour. Air may also contain traces of impurities such as carbon monoxide (CO), sulphur dioxide (SO2), hydrogen sulphide (H2S) and other gases. The presence of these gases in air results in air pollution. Table bellow shows the composition of air by volume. The proportion of water vapour and impurities in air is very variable.
The Gases Present in Air and their Proportions
The composition of air is not exactly the same everywhere. It changes
slightly from day to day and from place to place. There is more water
vapour
in the air on a damp day and in air above water bodies such as oceans,
seas, lakes, rivers, etc. Over busy cities and industrial areas
there
is more carbon dioxide. But the uneven heating of the earth’s surface
by the sun causes the air to move continually, resulting in
winds. The resultant winds spread the pollutants around.
The percentage composition of air by volume
| Gas | Approximate percentage |
| Nitrogen | 78.00% |
| Oxygen | 21.00% |
| Noble (rare) gases mainly argon | 0.94% |
| Carbon dioxide | 0.03% |
| Water vapour | 0 – 4% |
The Presence of Different Gases in Air
The determination of air by mass was carried out by Dumas in 1841. The apparatus used consists of three units as shown bellow.
The three parts of the apparatus include the following:
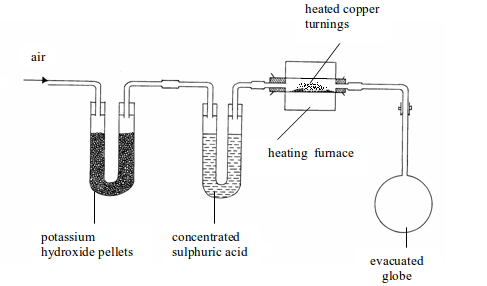
Determination of the composition of air by weight
- Several U-tubes containing potassium hydroxide pellets to remove carbon dioxide (only one tube shown in the figure for simplicity).
- Another set of U-tubes containing concentrated sulphuric acid to remove water vapour (only one tube shown in the figure).
- A heated, weighed glass tube containing finely divided copper to absorb oxygen.
The three parts of the apparatus would, therefore, remove all carbon
dioxide, water vapour and oxygen contained in air. The remaining gas
which
enters the weighed evacuated flask (globe) will be atmospheric nitrogen
and, of course, plus the rare gases. The copper will have
reacted
with all oxygen to form copper (II) oxide. The increase in mass of the
copper will give the mass of oxygen. The increase in weight of
the
globe will be due to the weight of nitrogen and the rare gases. If we
neglect the weight of carbon dioxide, the percentage of oxygen by
mass (weight) in dry, pure air is 23.2% and the remaining 76.8% is the percentage of nitrogen and rare gases.
The Percentage of Oxygen in Air Experimentally
Determine the percentage of oxygen in air experimentally
1. Experiment. Determination of the presence and proportion of oxygen in air by combustion of a candle
Method
- Place a small candle on a plastic lid or any object that can float. Then set up the apparatus as shown in figure bellow. Sodium hydroxide is used in order to absorb the carbon dioxide gas produced by a burning candle.
- Light the candle and place the measuring cylinder over the top. Note the level of sodium hydroxide solution in the measuring cylinder at the start. A candle will stop burning (go off) once all the oxygen in the cylinder is used up.
- When the candle goes off, leave the apparatus to cool to room temperature. The purpose of cooling is to let the heated and expanded air to return to its normal condition. Then note the level of sodium hydroxide solution in the measuring cylinder.
.
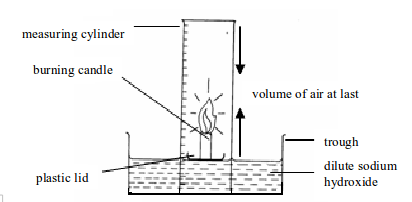
Determining the presence and percentage composition of oxygen in air by burning a candle
The oxygen in air enclosed in the measuring cylinder is used to burn the candle to produce carbon dioxide gas. The carbon dioxide so produced dissolves in sodium hydroxide solution. The dissolved carbon dioxide causes the level of sodium hydroxide solution to rise up. The oxygen gas used to burn the candle is practically equal to the amount of carbon dioxide produced. This fact is, therefore, used to calculate the percentage of oxygen in air.
In the experiment, the initial volume of air was found to be 70.5 cm3 and the final volume was 55 cm3. The percentage of oxygen in the air is calculated in two steps:
1. To find the volume of oxygen used up to burn the candle (which is
practically equal to the volume of carbon dioxide produced and then
absorbed
by sodium hydroxide), we subtract the final volume of air from the
initial volume Volume or oxygen used = Initial volume of air – final
volume of air
Therefore, the volume of oxygen used for combustion of the candle = 14.7 cm.

Alternatively,the volume of oxygen used up can be
calculated by subtracting the initial volume of sodium hydroxide
solution from the final volume. That is: Volume of oxygen used = final
volume of sodium hydroxide – initial volume of sodium hydroxide = Volume
of carbon dioxide dissolved in
sodium hydroxide.
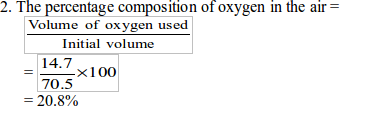
Therefore, the percentage of oxygen =
In practice, it is difficult to get an accurate result with the above experiment.

This is due to a number of reasons such as:
- Not all the carbon dioxide is absorbed by the sodium hydroxide.
- The candle may go out (stop burning) before all the oxygen is used up due to accumulation of carbon dioxide in the cylinder.
- The heating of the air inside the measuring cylinder causes the gases to expand. This is why it is essential that the gases be allowed to cool to room temperature before reading the level.
Experiment with combustion of copper in air gives the more accurate results than the combustion of the candle. The copper
reacts with oxygen in the air to give copper (II) oxide.
2. Experiment . Determination of the presence and proportion of oxygen in air by the combustion of copper in air
Method
- Set up the apparatus as shown in figure bellow. Syringe A should contain 100 cm of air, syringe B should be empty.
- Heat the copper strongly and pass the air from syringe A back and forth (by pushing the piston of the syringe inward and outward) over the copper turnings a few times. Allow the air to cool and measure the volume of air in syringe A.
- Repeat the heating and cooling until the volume of air that remains in syringe A is constant. The copper is heated and cooled several times to ensure that it reacts with all oxygen in the sample of air.
`
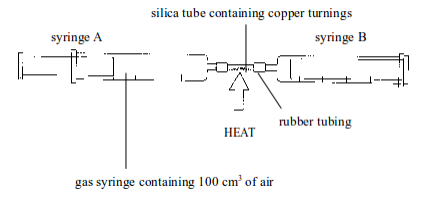
Determining the presence and percentage composition of oxygen in air by heating copper
Observations and findings
2. The final volume of air in the syringe, at the end of the
experiment, is less than that of the original volume. This is because
oxygen in the
original air has combined with copper
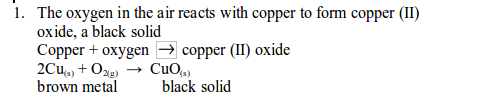
The volume of air in the syringe at different heating and cooling is as shown below:
Initial volume before heating = 100
Volume after first heating and cooling = 82
Volume after third heating and cooling = 79
The volume of oxygen used up = Initial volume of air before cooling – volume of air after the last heating and cooling
The presence of carbon dioxide in air
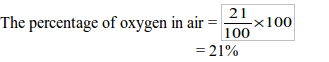
Carbon dioxide is present in air to the extent of 0.03% by volume. The gas is formed during the combustion of all common fuels – wood, coal, coke, natural gas, petrol, diesel, paraffin oil, etc, all of which contain carbon.
It is breathed out as a waste product of respiration by all animals. All sorts of combustion and burning produce carbon dioxide. The gas produced by all these processes accumulates in air. However, the amount of carbon dioxide in air remains constant instead of the tremendous quantities released into the atmosphere. This is because plants take up carbon dioxide. They then convert it into complex starchy compounds during photosynthesis. The gas also dissolves in ocean water and other water bodies.
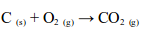
The presence of carbon dioxide in air can be shown by passing air through a test tube containing some lime water (figure 6.5). After a time, the lime water turns milky. This shows the presence of carbon dioxide.
The reaction involved is as follows:
“
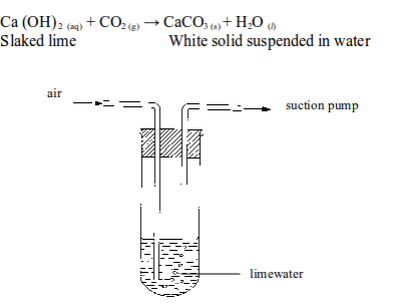
Testing for the presence of carbon dioxide in air
The presence of water vapour in air
Water vapour is present in air in varying quantities. It is given off
by evaporation from the oceans, lakes and rivers. The presence of water
vapour
in air can be demonstrated by exposing deliquescent substances to the
air on a watch glass. These are substances which when exposed to air
tend to absorb much moisture from the air, dissolve in that moisture,
and finally form a solution. Examples of deliquescent
substances include calcium chloride, sodium hydroxide and phosphorous pentoxide.
The resulting solution is distilled. The colourless liquid obtained
from distillation may be proved to be water by various water tests such
as
use of cobalt chloride paper or anhydrous copper (II) sulphate.
The cobalt chloride paper turns from blue to pink in the presence of
water.
The white anhydrous copper (II) sulphate turns blue. Any of the two tests confirms the presence of water.
Alternatively, one may expose the anhydrous copper (II) sulphate salt to open air straight away for quite some time and then observe any change in its colour and/or form. Upon absorption of water vapour from the air, the white, powdery and anhydrous copper sulphate salt turns into hydrated blue crystals.
About 1% of the air by volume is made up of the noble gases. The most abundant of the noble gases is argon. Others are neon, xenon, krypton and helium. The proportion of these four is very minute. Argon and neon are used in “gas-filled” electric light bulbs and coloured “neon” electrical signs. They are obtained from liquefied air.
The air always contains small quantities of many gases. Such gases include hydrogen sulphide, sulphur dioxide, as well as dust and other solid particles, especially in industrial areas. These gases are given off during the combustion of coal, and the fuels resulting from coal.
SEPARATION OF AIR INTO ITS CONSTITUENT GASES
The air we breathe is necessary to keep us alive. It is also a chemical resource. Oxygen is used in steel making, and nitrogen is used in making fertilizers. To use these gases in this way, they must be separated from the atmospheric air. Air, as we studied in chapter 5, is a mixture of different gases. The method used to separate its constituent gases is fractional distillation. The gases have to be liquefied so that the mixture can be fractionally distilled.
The process of separating the air into its constituent gases is difficult. It cannot be done in the laboratory. It is only done in industry. The chemical industry needs the gases from the air in their pure form.
The fractional distillation of air involves essentially two stages:
- First, the air must be cooled until it turns into a liquid.
- Then, the liquid air is allowed to warm up again. The various gases boil off at different temperatures
Stage 1: Liquefaction of air
- Air is filtered to remove any dust particles (purification).
- The air is cooled to -180°C to remove the water vapour and carbon dioxide.
- The air is then compressed to 100-150 atmospheres. As the compressed air gets very hot, it has to be cooled.
- The compressed cooled air is allowed to expand rapidly. The rapid expansion cools the air to very low temperatures, and the liquid drops out. At -200°C, only helium and neon remain as gases. The cold gases are used to cool the compressed air.
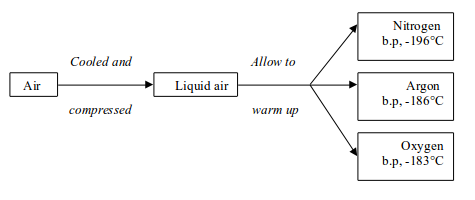
Stage 2: Fractional distillation of liquid air
The air is cooled and compressed to form liquid air. The liquid air
is allowed to warm up. Nitrogen boils off first because it has a low
boiling point, -196°C. Argon follows by boiling at -186°C and finally oxygen at -183°C
Figure above illustrates all the steps that take place during the process.
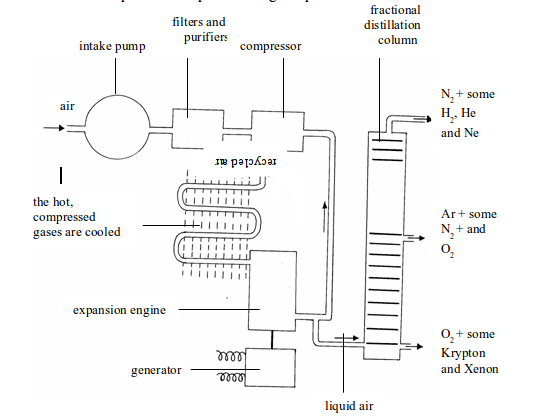
Fractional distillation of liquid air
Rusting is the name given to the oxidation of iron or steel in damp air. It is also called corrosion. Rust is hydrated iron (III) oxide. It is a soft, crumbly solid and hence weakens the structure of iron and steel. During rusting, iron reacts with oxygen to form brown iron (III) oxide
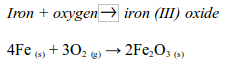
At the same time the iron (III) oxide reacts with water to form hydrated iron (III) oxide (or rust):
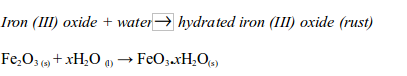
Note: The x in the equation indicates that the number of water molecules in the hydrated iron (III) oxide can vary. So, both oxygen and water are needed to cause rusting of iron.
Rusting is a serious economic problem. Large sums of money are spent each year to replace damaged iron and steel structures, or protecting structures from such damages. Rusting of bridges, corrugated iron sheets on house roofs, containers, articles, etc. require an expenditure of big sums of money as well as labour for replacement. Rust weakens structures such as car bodies, iron railings, and ships’ hulls, and shortens their useful life. Preventing it can cost a lot of money. All efforts must be made to stop iron or steel items from rusting. This can be achieved if we know the conditions necessary for iron to rust.
The Conditions Necessary for Iron to Rust
When iron is left in contact with both water and oxygen (or air), it
reacts to form hydrated iron (III) oxide. Iron will not rust on exposure
to dry air or air-free water (water that has been boiled to expel all
dissolved air) only. However, iron will easily and readily rust in water
that
has dissolved air in it. In figure 6.8, only the iron nail that is in
contact with both water and air rusts. Therefore, rusting will only
occur
in the presence of both water and oxygen. If one of the two conditions
is excluded, in one way or another, rusting will not take
place at all.
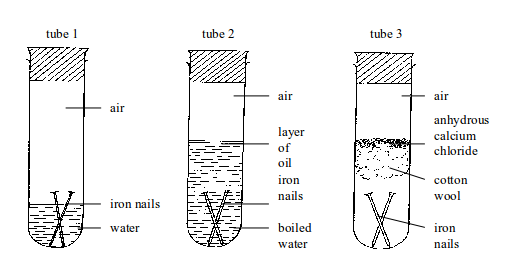
Testing for conditions necessary for iron rusting
Nails in tube 1 will rust. Nails in tubes 2 and 3 will not rust.
In tube 1, nails are in contact with both water and air (oxygen).
In tube 2, the water has been boiled to expel the
dissolved air. In addition, any air above the water is prevented from
dissolving in boiled water by a layer of oil. So, the nails are
completely shielded away from air.
Therefore, rusting is impossible.
In tube 3, nails are in contact with air only. The moisture present in
air is absorbed by anhydrous calcium
chloride. Any moisture that
might have been absorbed by the anhydrous calcium chloride is prevented
from reaching the nails by a tuft of
cotton wool. The cotton wool
also absorbs some moisture directly from the air. Therefore, tube 3 will
always carry dry air (moisture-free
air). Hence, no rusting of iron nails occurs.
This experiment demonstrates the fact that for iron to rust, both water and air (oxygen) must be present. If one of these conditions is controlled, no rusting can take place.
Similarity between rusting and burning
Chemically, rusting and burning are similar processes in that they both require oxygen. Consider the burning of magnesium to give magnesium oxide.
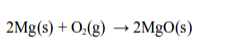
In this process, magnesium combines with the oxygen of the air to form magnesium oxide.
During rusting, iron combines with oxygen of the air in the presence of water to form brown hydrated iron (III) oxide, “rust.”
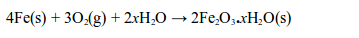
In addition, the two processes, burning and rusting, are exactly
similar in that they both generate heat. The only difference is in the
time
required for each of the two processes to take place. During
rusting heat is given out, but without being noticed because of its
slower rate
of production. Burning produces noticeable heat and light.
The Different Methods of Preventing Iron from Rusting
We have learned that for iron to rust there must be direct contact between the iron and both water and oxygen from the air. Therefore, in order to stop rusting we must protect iron from either water (moisture) or oxygen (air) or both. The following are some of the methods used to prevent iron from rusting:


No comments:
Post a Comment