
Introduction to Organic Chemistry
All organic compounds contain carbon together with one or more other elements such as hydrogen, oxygen, nitrogen, sulphur and the halogens.
Normally every compound of carbon is an organic compound. Even after discovering that these compounds could be synthesized in the laboratory, the definition that they are organic (of organic nature, that is, they originate from living things) has been retained. However, for
conventional and historic reasons, some of the simpler compounds such as carbon dioxide (CO2) carbonates, carbon monoxide (CO3) are usually studied with other non-carbon compounds in Inorganic Chemistry.
Difference between Organic from Inorganic Chemistry
Organic chemistry is the chemistry of carbon compounds. All organic compounds contain carbon and other elements such as H, O, N, S and the halogens.
Normally every compound of carbon is an organic compound. Examples of organic compounds/substances are plastics, milk, carbohydrates, lipids, proteins, sugar and hydrocarbons. Inorganic substances includes table salt, CO2, diamond, iron and water.
The Importance of Organic Chemistry in Life
Carbon is the most unusual atom. It has the ability to join up to itself and form very long chains of atoms. Without this ability, life on Earth would not exist because the molecules that make our bodies contain mostly long chains of carbon atoms.
All living things contain carbon compounds. Raw materials such as oil and coal, derived from living things, are also based on carbon. Our modern society is very much dependent on organic chemistry to make the fuels and materials that we use in every day of our lives.
In particular, polymers, large molecules obtained from alkanes, have very widespread use. Without alkanes from crude oil our transport system would be impossible.
We also need various fractions obtained from crude oil (petrol, diesel, kerosene, oil, natural gas, etc.) to run motor vehicles and other machines to simplify our work and life.
In brief, the compounds obtained from crude oil have thousands of different uses, for example:
- some are used as fuel or converted into fuels;
- some are used for making detergents, dyes, drugs, paints, and cosmetics; and yet some are used for making polyethene, polyvinyl chloride (PVC) and other plastics.
The Origin of Organic Compounds
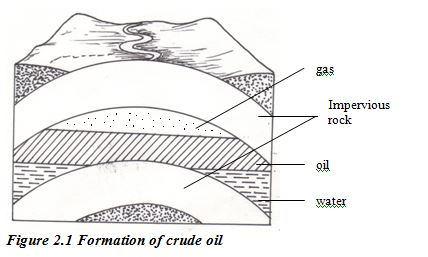
Fossil fuels were formed in the Earth’s crust from material that was once living. Coal comes from fossils of plant material. Crude oil and natural gas are formed from bodies of marine microorganisms. The formation of these fuels took place over geological periods of time (many millions of years).
Crude oil is one of the world’s major natural resources. The oil is the result of a process that began up to 400 million years ago. Prehistoric marine organisms died and sunk to the sea bed and were covered by mud and sand.
The change into crude oil and natural gas was brought about by high pressure, high temperature and bacteria acting over millions of years. The original organic material broke down into hydrocarbons.
Compression of the mud above the hydrocarbon mixture transformed it into shale. Then geological movements and pressure changed this shale into harder rocks, squeezing out the oil and gas. The oil and gas moved upwards through the porous rocks, moving from high- pressure to low-pressure conditions.
Sometimes they reached the surface, but often they became trapped by a layer of non porous rock. Reservoirs of oil and gas were created. These reservoirs are not lakes of oil or pockets of gas. Instead, the oil or gas is spread throughout the pores in coarse rocks such as sandstone or limestone the same as water is held in a sponge.
The Fractional Distillation of Crude Oil
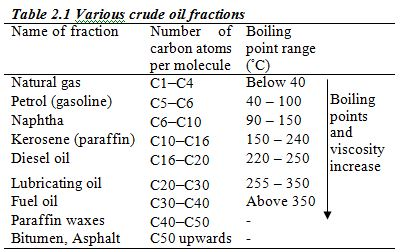
Crude oil from an oilfield is separated from impurities such as sand and water and is pumped through pipelines to the refinery. At the refinery, fractional distillation is used to separate the crude oil into several fractions, each fraction containing several hydrocarbons which boil within a certain range of temperatures.
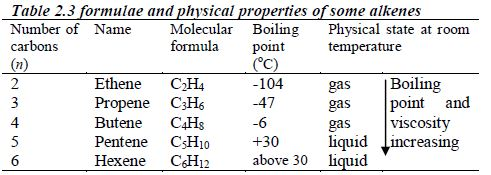
These different boiling points are roughly related to the number of carbon atoms in the hydrocarbon (Table 2.1)
Separation of the hydrocarbons takes place in a fractionating column (fractionating tower). At the start of the refinery process, crude oil is preheated to a temperature of 350–400°C and pumped in at the base of the tower. As it boils, the vapour passes up the tower. It passes through a series of bubble caps and cools as it rises further up the column. The different fractions cool and condense at different
several different levels, the higher the level, the lower the boiling point of the fraction removed. Figure 2.2 shows the process of
separation of crude oil into different fractions.
After fractional distillation, impurities are removed. The commonest impurity is sulphur, which is removed and used to manufacture sulphuric acid. If petrol (gasoline) containing sulphur is not purified before it is used in an internal combustion engine, the exhaust fumes will contain oxides of sulphur (SO2 and SO3). These are poisonous gases and will pollute the environment.

Uses of different petroleum fractions
- 1. Natural gas (refinery gas). The gas fractions consist of mainly methane, ethane, propane, and butane. The methane and ethane are usually burnt as fuel. The propane and butane are liquefied and are distributed in high pressure gas cylinders and tanks to be used for lighting and heating purposes.
grease stains and paints.
machines.
Hydrocarbons
Hydrocarbons are compounds containing hydrogen and carbon and no other element. That is, a hydrocarbon has the molecular formula CxHy, x and being whole numbers. For example, methane (CH4) ethane (C2H2) and benzene (C6H6) are hydrocarbons.
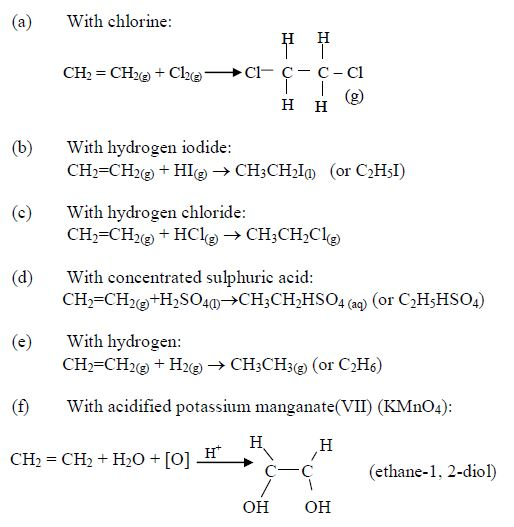
The three Families of Hydrocarbons
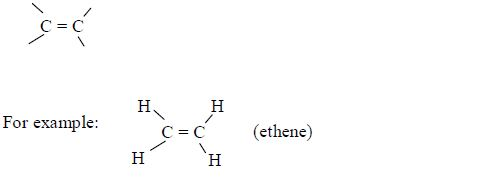
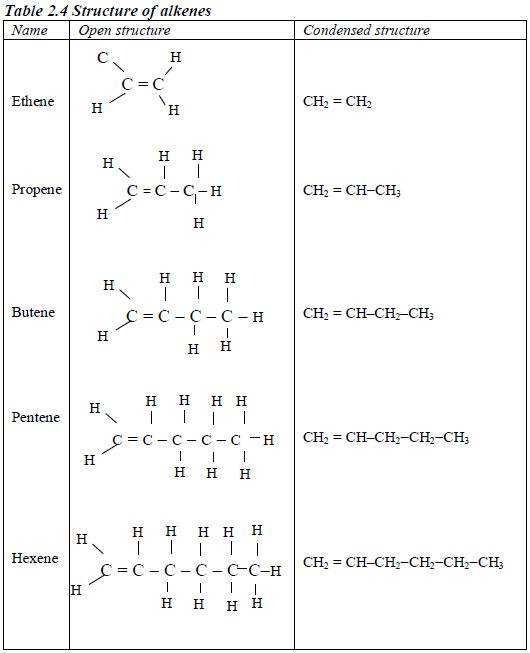
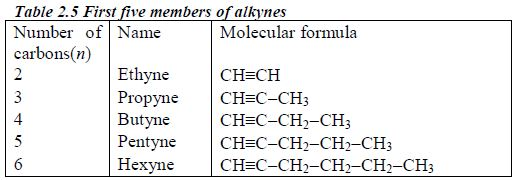
In order to simplify the study of these compounds, chemists have grouped them into families. The members of each family have characteristic chemical properties and graded physical properties, such as boiling point, etc. The main family members of hydrocarbons that we shall study at this level are the alkanes, the alkenes and the alkynes.
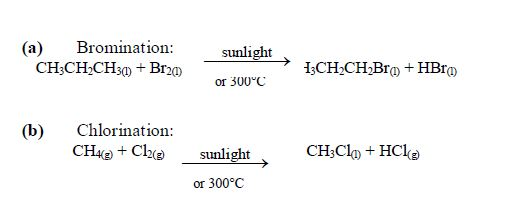
Alkanes
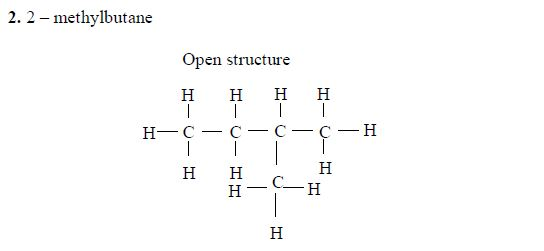
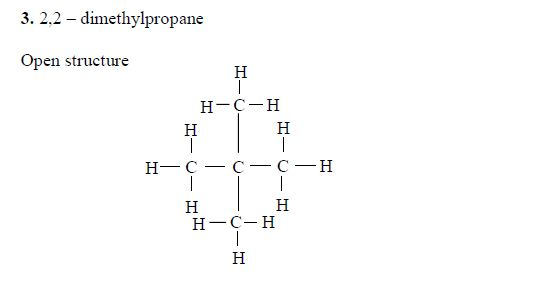
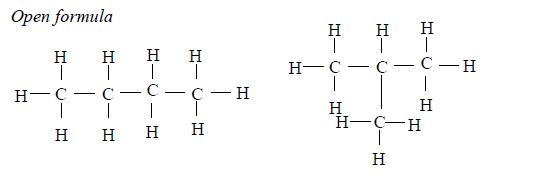
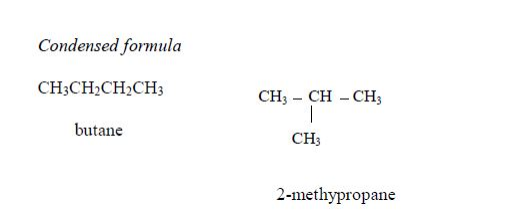
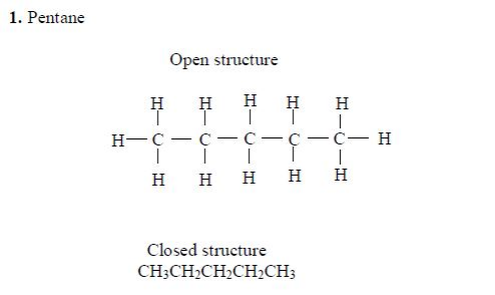
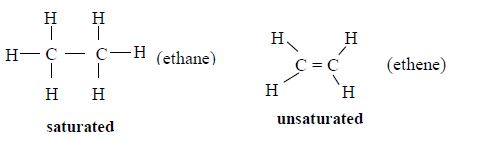
The members of this group of hydrocarbons are distinguished by possessing the general molecular formula Cn H2n+2, where is 1, 2, 3, etc., for successive members of the group. The first member of the series (n = 1) is methane (CH4) and the second (n = 2) is ethane (C2H6).
Both are gases at room temperature and pressure. This general formula can be used to work out the formula of any other alkane if we know the number of carbon atoms it consists of. The following table gives the molecular formula and name of the first few alkanes, plus an indication of some of their physical properties.
Carboxylic Acids
Natural Sources of Organic Acids
- milk (lactic acid)
- citrus fruits (citric acid);
- tobacco (nicotinic acid); and
- tea (tartaric acid).
The Oxidation of Ethanol to Ethanoic Acid
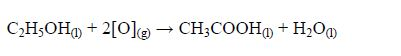 The Structures of the Homologues of Carboxylic Acids up to Five Carbon Atoms
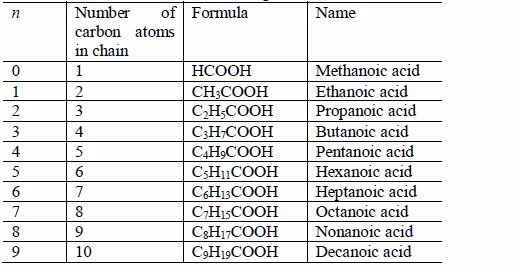
The Structures of the Homologues of Carboxylic Acids up to Five Carbon Atoms
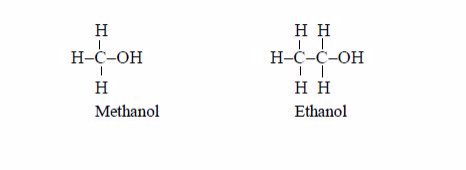
The two lowest members, containing one atom and two carbon atoms respectively, are:

The Isomers of Carboxylic Acids up to Five Carbon Atoms
Rules
The carbon of the carboxyl group (–COOH) is considered as carbon atom number
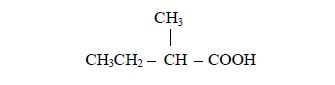
2. Name the branched alkyl group, followed by the name of the acid to which the alkyl group is attached. For example, in the case above (rule no.2):
- the alkyl group is methyl;
- it is attached to carbon number and
- the acid to which it is attached is butanoic acid,CH3CH2CH2COOH
be used. For, example in the compound
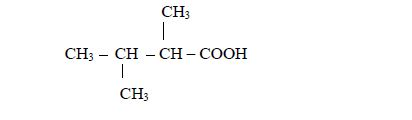
- there are two methyl groups, one attached to carbon number 2 and the other to carbon number 3; and
- they are both attached to butanoic acid chain.
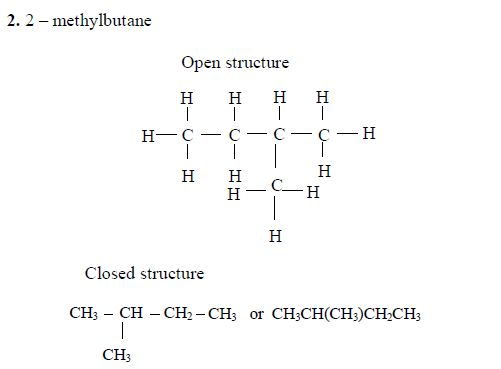
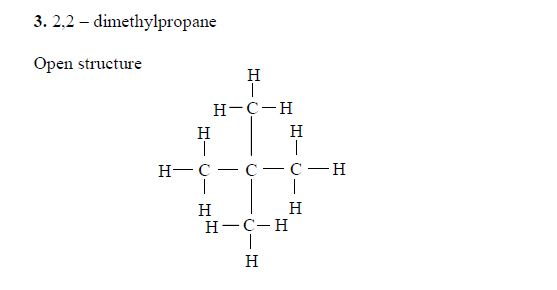
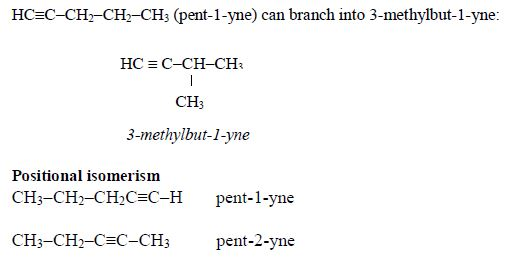
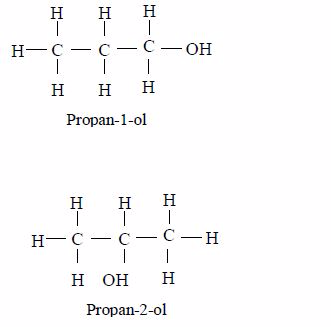
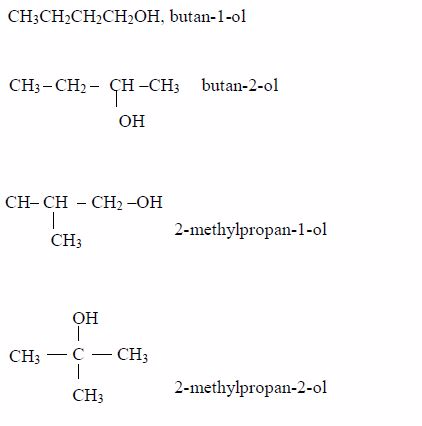
Isomerism and nomenclature
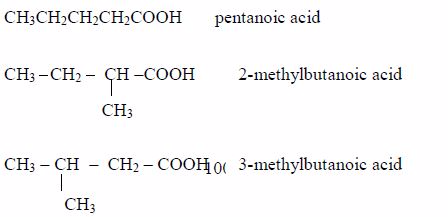
The first three members of the series do not show isomerism because their hydrocarbon ends do not form branches. The following are the structures and names of the isomers of carboxylic acids up to five carbon atoms:
- Butanoic acid, C3H7COOH or C3H7CO2H or CH3CH2CH2COOH
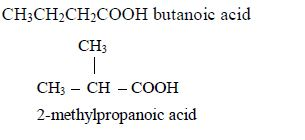
- Pentanoic acid, C4H9COOH
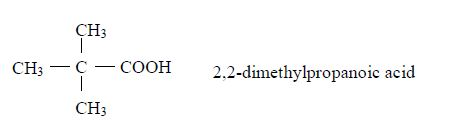
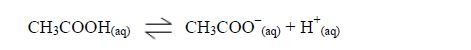
affect indicators, just like the inorganic acids do.
Neutralization

The reaction between carboxylic acids
and alcohols is called esterification. The acids will react reversibly
with alcohols to form
sweet–smelling esters. Concentrated sulphuric acid is a catalyst for the reaction.
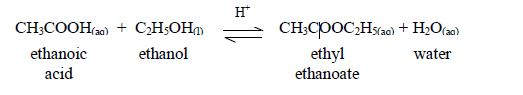
Esters are manufactured for use as solvents, food flavourings, and fragrance for perfumes and beauty products. Ethyl ethanoate is just one example of many esters. The esters usually have strong and pleasant smells.
Many of these compounds occur naturally. They are responsible for the flavours in fruits and for the scents of flowers. Fats and oils are naturally occurring esters used for energy storage in plants and animals.
Some of the naturally occurring esters include:
- vegetable oils e.g. palm oil, groundnut oil, cashewnut oil, olive oil, sunflower oil, etc; and
- animal fats.

Preparation of Soap from Animal Fats or Vegetable Oil
They are called “fatty” because the long chains repel water, making them immiscible with water. Glycerol or glycerine (or propane–1,2,3-triol) has three –OH groups. This is how fatty acids and glycerol react:
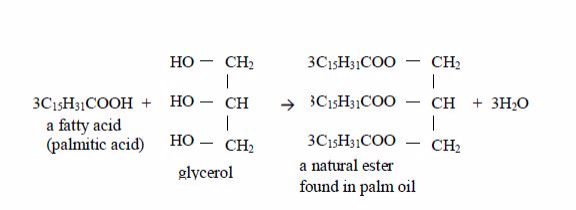
Preparation of soap from oils
Soap is made by heating animal fats or vegetable oils with sodium hydroxide solution. The oils react with the solution of sodium hydroxide and break down to form glycerol and the sodium salts of their fatty acids. These salts are used as soap. The reaction equation is:
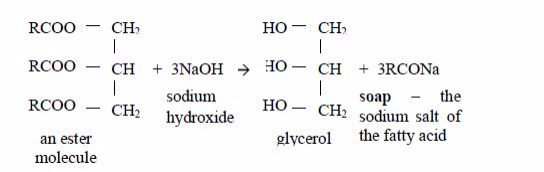
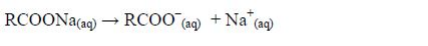
The cleansing agent in soap is the ion, RCOO–

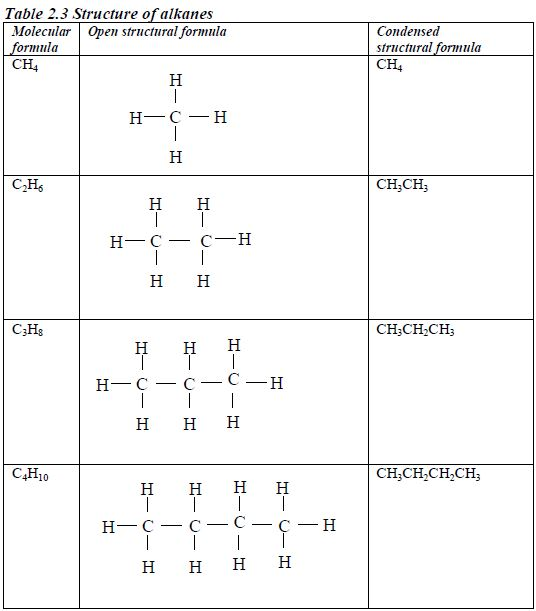
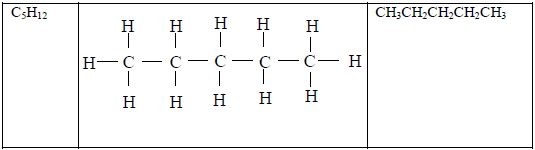
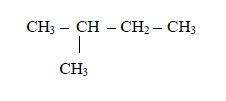
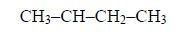
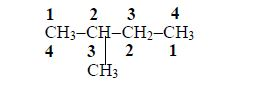
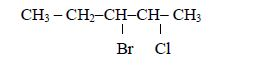
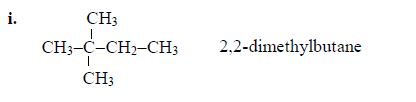
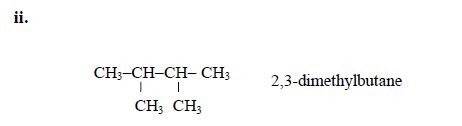
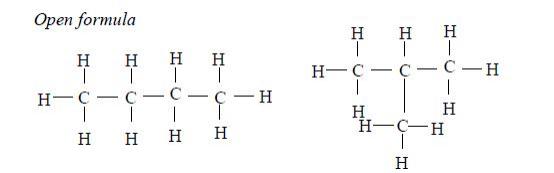
 Example 2
Example 2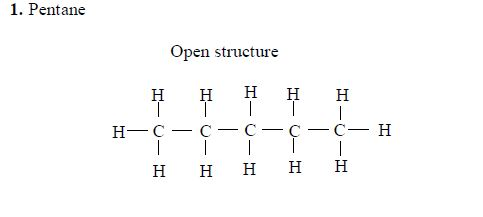

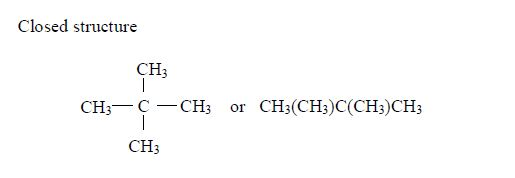
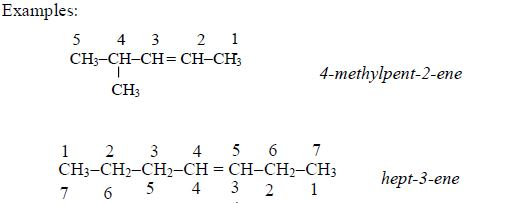
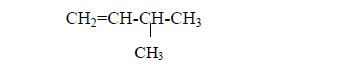
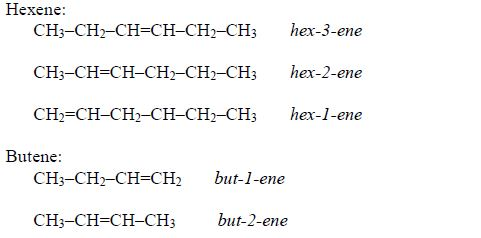
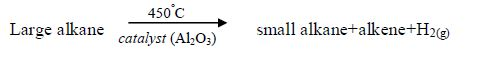
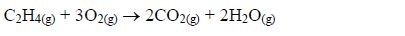
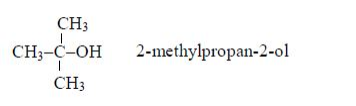
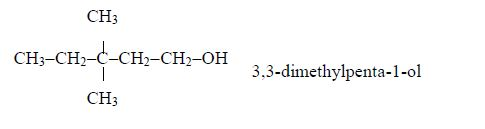
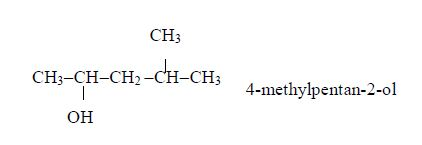
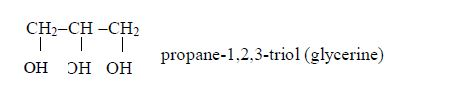

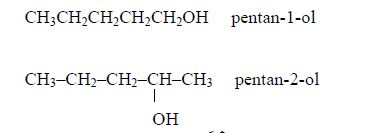

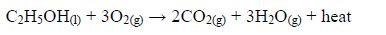
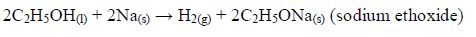
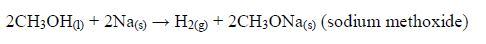
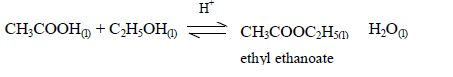
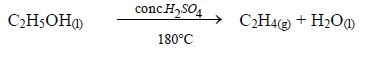
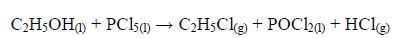
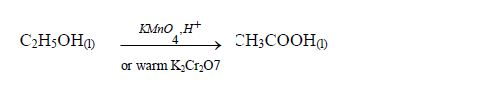

No comments:
Post a Comment