
TOPIC 3: SOIL CHEMISTRY | CHEMISTRY FORM 4
Soil Formation
Soil is formed by the process of weathering. All types of weathering (physical, chemical or biological) result to disintegration of rocks into smaller particles. Air and water enter the space between these particles and chemical changes take place, which lead to the production of chemical substances.
Bacteria and plant life soon appear. When plants and animals die, they decay and produce humus. Bacteria and other decomposers play a vital role in the decomposition of plant and animal substrata. The end product of these mechanical, chemical and biological processes is soil.
All soils contain mineral matter, organic matter, water, air and living organisms, especially bacteria. If any one of these is substantially reduced in amount or is removed from the soil, then the soil deteriorates.
There are many types of soil and each has specific
characteristics related to the climate, the vegetation and the rock of
the region in which it forms. The weathering processes of a region also
play an important part in determining soil characteristics. The relationship of these factors is as shown in figure 3.1.

The Factors Influencing Soil Formation
Information about soil formation can lead to better soil classification and more accurate interpretation of soil properties.There are several factors responsible for soil formation.
The factors include climate, living organisms, relief (topography),parent material and temperature. All the factors, except time,depend to a greater or lesser extent upon each other, upon the soil itself or upon some other factor. None of the factors can be considered more important than any other, but locally one factor may exert a particular strong influence. These factors are explained in details below.
1. Parent material
Some rocks are more easily weathered than others. Acidic rocks are more resistant to weathering than basic rocks. The parent rock affects soil texture and water permeability.
2. Climate
vegetation. The dead vegetations decay to form humus as one of the components of the soil.
To understand well the influence of climate on soil formation let us have a look at its components and how each of these components affects soil formation.
3. Temperature
The main effect of temperature on soil
is to influence the rate of reactions; for every 10°C rise in
temperature, the speed of a chemical
reaction increases by a factor of 2 or 3 (twice or thrice). Temperature,
therefore, influences the speed of disintegration and decomposition of
the parent materials and its consolidation to form the soil.
4. Rainfall (water)
The water in soils includes all forms of
water that enter the soil system and is derived mainly from
precipitation as rain. The water entering
soils contains appreciable amounts of dissolved carbodioxide, forming a
weak carbonic acid. This dilute, weak acid solution is more reactive
than pure water. It thus reacts with unconsolidated minerals and organic
matter, breaking them down into mineral (clay, sand) and organic debris
(humus) respectively.
5. Organisms
organisms of interest to soil formation are as follows:
binding together small groups of particles hence developing a crumby or granular structure. Large roots are agents of physical weathering as they open and widen cracks in rocks and stones. When plants die they contribute organic matter to the soil, which acts as a binder of the
soil particles. Higher plants intercept rain and they shelter the soil from the impact of raindrops. They also shade the soil and hence reduce
evaporation.
Mesofauna
- ingesting organic mineral materials e.g. earthworms and millipedes;
- transportation of materials e.g. earthworms, millipedes, termites, beetles, etc; and
- improvement of soil structure and aeration.
- Cultivation of soils for production of food and tree crops, which in many cases has negative effects causing impoverishment of the soil and erosion.
- Indiscrimate grazing, casual burning, cutting of trees, manure and fertilizer use, all of which alter the soil characteristics.
6. Relief (Topography)
solution through the roots.
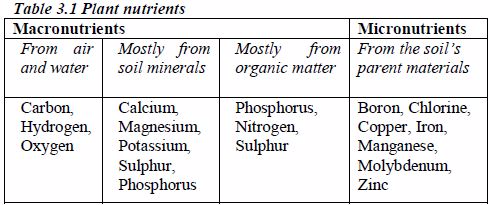
Nitrogen is taken in as ammonium or nitrate (V) ions; phosphorus as dihydrogenphosphate (V) ions; sulphur as sulphate (VI) ions and chlorine as chloride ions.
proportion to facilitate optimum plant growth.
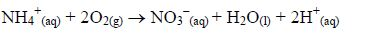
It is used to build amino acids, nucleic acids, many enzymes, chlorophyll, generally speaking: all proteins.
It promotes the vegetative growth in plants. It is therefore important in the growth of plants in which leaves are harvested, such tobacco and vegetables.
It is an essential element in cell division. It is therefore needed for plant growth.
It increases grain size and protein content in cereals.
It promotes root growth.
fertilizers must be carefully dosed.
Phosphorus is an essential component of the genetic material of the cell nucleus (RNA, DNA); also in ADP and ATP, which play a vital role in photosynthesis, amino acid and fat metabolism, etc.
It increases the grain yield e.g. of millet, sorghum and rice because it promotes the formation of tillers.
It promotes root growth.
It strengthens the resistance of plants to diseases.
Also rhizobia bacteria need it in order to fix nitrogen from the air.
It hastens plant maturity.
Dark green colouration
Purple spots or streaks.
Stunting, delayed maturity.
It does not form any integral part of the structure of any known organic compounds in plants.
Potassium is an activator of a number of enzymes involved amino acid synthesis and several enzymes concerned with carbohydrate and nucleic acid metabolism.
Potassium aids in the uptake of other nutrients and in their movements within the plant e.g. potassium ions and nitrate (V)
ions may move together.
Potassium is also important in the metabolism of carbohydrates and translocation of food. Thus, it promotes starch and sugar formation.
It regulates osmosis in cells, improves tissue formation and assists in protein synthesis.
It strengthens plant stalk, hence preventing lodging and microbial attack.
Stunting: First the edges of the older leaves and then areas between veins turn yellow and finally brown. Small, brown necrotic spots develop while the veins are still green.
Leaf curling and premature leaf fall.
Calcium is a constituent of cell walls and hence makes the straw stiff and resistant to lodging.
It is essential for cell division.
It promotes early root and seed development.
It regulates the intake of potassium by plants.
It neutralizes harmful organic acids like ethanedioic (oxalic) acid in plants, thus detoxifying them:
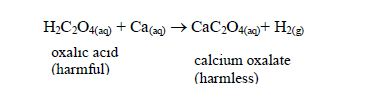
Magnesium is vital to the production of chlorophyll, because every molecule of chlorophyll contains a magnesium ion at the core of its complex structure. Most of the magnesium in plants is found in either chlorophyll or seeds. A lesser part is distributed in other parts.
Aids in the translocation of carbohydrates.
Regulates the uptake of other nutrients.
Part of the distributed magnesium functions in the enzyme system involved in carbohydrate metabolism.
Sulphur is a vital part of plant proteins since cystine and methionine are sulphur-containing amino acids.
Sulphur is also essential for the action of enzymes involved in nitrate (V) production.
In igneous rocks, iron occurs in the Fe2+ form. The iron in water-logged soils tends to remain in this form and contributes to the
bluish-grey colours that indicate wetness. Much of the iron in well drained soils is in the Fe3+ form and is associated with humus and
mineral particles.
Iron (II) sulphate (FeSO4) which is soluble in water. Application of iron is generally ineffective to calcareous soils.
Micronutrients are taken in by plants as Bo2–, Co2+, Cu2+, Mn2+, MoO2– and Zn2+. The micronutrients in the soil usually originate from the parent material of the soil. Plant needs of these micronutrients are very small.
Manganese is a catalyst in the formation of chlorophyll and in many redox reactions, e.g. metabolism of nitrogen, iron, copper, zinc and in vitamin C synthesis.
Boron aids protein synthesis, regulates the K:Ca ratio in plant tissues and is required for the formation of roots and fruits.
Copper is involved in respiration and in the nitrogen and iron metabolism.
Molybdenum is essential in the protein synthesis and for the nitrogen fixation by rhizobia on the roots of legumes.
Zinc catalyses the formation of growth hormones and promotes the synthesis of RNA and chloroplasts. Thus it is essential for normal growth.
Chlorine seems to be essential in photosynthesis and is required for plant growth.
Cobalt is essential for nitrogen fixation by rhizobia and hence aids growth of legumes. However, it is not clear whether it is essential for growth of higher plants.
Large amounts of micronutrients are usually toxic to plants. The best method of application is usually foliar spraying.
(replaced). There are several methods that when combined at least in some aspects can help raise or maintain soil fertility. These are:
However, if used without proper knowledge or advice by agricultural officers they can be harmful to the soil, crops, animals and humans.
biogas manure – from biogas plants
farm yard manure – from wastes of farm animals such as cattle, sheep, goats, poultry, pigs, donkeys, etc;-
compost manure – from decomposed organic matter; and-
leguminous green manures, like sunhemp, beans, cowpeas, groundnuts, peas, etc.
These young plant materials when ploughed and incorporated into the soil provide organic matter and nitrogen.
conservation measures which include mulching terracing/ridging, deep tillage, contour ploughing, strip cropping, planting shelter belts or
windbreaks, reforestation, avoiding overgrazing and overstocking, etc.
leguminous crops (which fix nitrogen in the soil) followed by non-leguminous crops and vice-versa.
provide the cereals with the humus when their leaves fall and rot on the soil. They also provide forage for animals, and firewood. Agroforestry is also one of the protections against soil erosion.
stopped or reduced by maintaining adequate levels of soil organic matter to trap the nutrients and also by avoiding too much irrigation. It can also be stopped by avoiding overcultivation, a fact which makes the soil too loose that the nutrients are easily percolated with the soil
solution to the bottom soil layers making these nutrients unavailable to plants.
possible for farmers with plenty of land. It was an equally good practice in the past when human population was low as compared to
vastness of the land at that time. It is not widely practiced today except in areas with low population density and abundant arable land.
status. But if appropriate crops are planted in the right soil type, chances of maintaining or sustaining the fertility of that soil is also
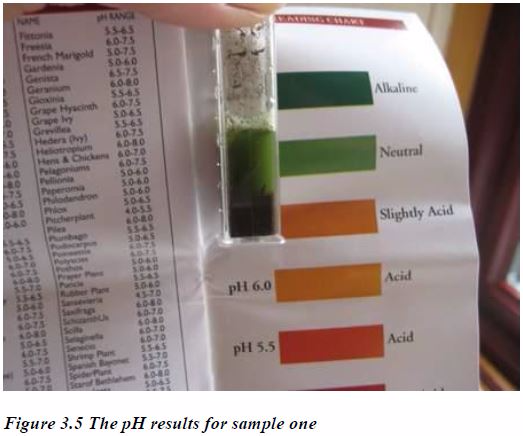
Likewise, an acid soil, no matter how much beneficial nutrients it contains, is of no use unless its acidity is corrected
These nutrients must be maintained in the soil by good cropping systems, application of appropriate fertilizers and manure and adopting good soil management practices.


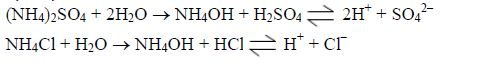
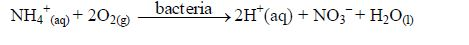

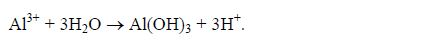






No comments:
Post a Comment