TOPIC 2: LABORATORY TECHNIQUES AND SAFETY
A laboratory is a room or building specially designed for conducting various scientific experiments.
An appropriate school laboratory has the following features:
- a room with enough space for carrying out scientific experiments;
- a store for keeping laboratory apparatus, chemicals and reagents;
- an office for laboratory technician to sit in and design scientific experiments;
- enough ventilation to let in fresh air and light;
- wide doors and several exits for emergency evacuation in case of an accident; and
- a wide table in front of the laboratory room, fitted with sinks for experiment demonstrations by the teacher or technician.
Rules and safety precautions in a chemistry laboratory
Chemistry is best studied through doing experiments. Most experiments are conducted in the laboratory. It is important to read and follow laboratory rules to avoid causing accidents. Your teacher will teach and give you more rules. The following are some important laboratory rules:
- Do not enter the laboratory without permission from your teacher or laboratory technician.
- Wear safety goggles all the time while in the laboratory. Obey this rule whether you are actually working on an experiment or simply writing in your laboratory notebook.
- Contact lenses are not allowed. Even when worn under safety goggles, various fumes may accumulate under the lens and cause serious injuries or blindness.
- Put on closed shoes and trousers when in the laboratory. Sandals and shots are strictly prohibited.
- Never walk or run unnecessarily in the laboratory.
- Tie back long hair when using open flames.
- Eating, drinking, and smoking are strictly prohibited in the laboratory.
- Don’t perform any experiment not authorized by your teacher or lab technician. If you are curious about trying a procedure not covered in the experimental procedure, consult your teacher or laboratory technician.
- Never taste anything. Never directly smell the source of any vapour or gas; instead drift a small sample to your nose. Do not inhale this vapour directly but take in only enough to detect an odour if one exists.
- Always wash your hands after experiments.
- Never use your hands to transfer chemicals. Use a spatula instead.
- Notify your teacher or technician immediately in case of an accident
- Know what chemicals you are using, carefully read the label twice before taking anything from the reagent bottle. Do not interchange labels.
- Excess reagents are never to be returned to stock bottles. If you take too much, dispose of the excess.
- Do not use anywhere near open flames highly flammable.Many common reagents, for example, alcohol, acetone and carbon disulphide are highly flammable. Do not use them anywhere near open flames.
- Pour more concentrated solutions into less concentrated solutions to avoid violent reactions. For example, always add acid to water; not water to acid. If you pour water into acid instead, the heat of reaction will cause the water to explode into steam, sometimes violently, and the acid will splash.
- If chemicals accidentally splash onto your skin or eyes, flush immediately with plentiful amounts of water and report to your teacher or lab technician.
- Never point a test tube or vessel that you are heating at yourself or your colleague.
- Dispose of chemicals properly. Unless you are told otherwise, assume that only water may be poured in the laboratory sinks.
- When an experiment is completed, always clean up your work area and dispose of the broken glass properly. Return all equipment to its proper storage places.
- Never take away anything from the laboratory without your teacher’s permission.
- Beware of hot glass because it looks exactly the same as a cold glass. Never touch it with your hand.
- Always adjust the Bunsen burner to give a luminous flame when not using it (or just simply turn it off).
- Use equipment or apparatus only for its designated use.
- Never eat or drink from laboratory glassware.
- Make sure all the burners are turned off before leaving the laboratory. Check that the gas tap is off as well.
- Never heat a liquid in a closed container. The expanding gases produced may blow the container apart, injuring you or others.
- Use only those chemicals needed in the activity. Keep all lids closed when a chemical is not used.
- Do not use the same spatula to remove chemicals from two different containers. Each container should have a different spatula.
- Replace all stoppers, covers and caps as soon as you finish using it. Be careful not to exchange stoppers from two different containers.
- When heating glassware, use wire gauze or ceramic screen. This will protect glassware from the flame of a Bunsen burner.
- Never use broken or chipped glassware. If glassware breaks, inform your teacher and dispose of glassware in the litter bin.
- Keep all windows open for proper ventilation.
- When carrying out the experiment where you expect harmful gases to be produced, use the fume chamber. The fume chamber helps to disperse hazardous gases and vapours safely.
- Use a lighter or wooden splint to light burners. Do not use papers. Always strike the match before turning on the gas supply.
- In case of a gas leakage, turn off the gas tap and open the windows. Leave the room immediately.
- Do not touch any electrical equipment with wet hands.
- Turn off any gas or water taps that are not in use.
The Safety Measures for a Chemistry Laboratory
The chemistry laboratory can be a place of discovery and learning. However, by the very nature of laboratory work, it can be a place of danger if proper common-sense precautions are not taken. Effort has been made to eliminate the use of explosives, highly toxic and carcinogenic substances from the experiments which you will perform. However, there is a certain unavoidable hazard associated with the use of a variety of chemicals and glassware. You are expected to learn and adhere to all safety guidelines. This will ensure a safe laboratory environment for yourself and the people you may be working with or those near you. The following are important laboratory safety measures to obey:
- Label and lock all storage areas, cupboards, drawers, storage
cabinets, refrigerators, etc. Locking will prevent accidental contact
with
chemicals or interference with equipment. - Be familiar with the location, use and limitations of the safety devices. This includes fire extinguishers, fire blankets, fume hood, spill cleanup materials, first aid kit, eyewash stations and fire alarm.
- Keep all chemicals in properly labelled containers. This will prevent accidental use of the wrong chemical for a particular experiment.
- Be familiar with the appropriate safety measures to take when exposed to different hazardous materials. Information is available from your teacher or laboratory technician.
- All chemicals that react with each other must be stored separately.
- Be
aware of the interaction of laboratory furniture and equipment with
chemicals used or stored in the laboratory. For example, oxidizers
should not be stored directly on wooden shelves. - Use fume hoods/cupboards/chambers whenever possible.
- Never store food in a refrigerator or freezer where hazardous chemicals are stored. Also, do not eat anything you find in the laboratory or in the laboratory freezer or refrigerator.
- Make sure fire extinguishers are in good condition. Report any broken seals, damage, low gauge pressure or improper mounting to the teacher or laboratory technician. If the seal has been broken, assume that the fire extinguisher has been used and must be recharged. (Note: Do not use fire extinguishers unless you are trained and feel confident to do so).
- Stored chemicals must be inspected regularly to ensure they have not expired. Note the date when bottles were received and when were first opened. Note expiry dates on chemicals and their special storage conditions.
- Eliminate safety hazards by maintaining laboratory work areas in a good state of order.
- The laboratory must have wide emergency exits and wide windows. Wide exits facilitate easy evacuation in case of emergency. Wide windows allow enough air to enter and circulate in the laboratory. (Note: Maintain at least two clear passages to laboratory exits).
- Always keep tables, seats, fume hoods, floors and desks clear of unnecessary material.
- All equipment should be inspected before use. In addition, they should be checked regularly to ensure they are safe for use.
- If experiments must be left unattended, place a note next to experimental apparatus indicating the chemicals involved, your name and telephone number on which you can be reached in case of an emergency.
- Keep
the laboratory floor clean and dry at all times. Clean spills of water
or chemicals immediately. Then notify other laboratory workers of
potential slipping hazards. - The laboratory must be equipped with potable fire extinguishers and other safety devices with clear instructions on how to use them in case of any emergency.
- Containers for holding or storing chemicals must be inspected for leakages or other damages. They should have tight stoppers or covers.
- All experimenters and other persons working in the laboratory should wear protective gears to minimize exposure to hazards. These gears may include lab coats, hand gloves, gumboots, safety goggles, aprons, etc.
- There should be a manual or instruction guides on how to treat spills of different chemical substances.
- The fume chamber should be labelled. It should be kept in good condition to minimize unexpected gas leakages or emissions.
- Gas cylinders should be labelled, stored properly, and supported. Moreover, they should be in good working conditions all the time.
- Each laboratory should be equipped with adequate first aid kits.
- Equipment for monitoring contamination should be installed to give alerts of any possible dangers.
NOTE:
All the above rules and safety measures are
applicable to all research, teaching and academic laboratories.
However, your laboratory may require some more rules that apply to
specific materials and equipment.
FIRST AID AND FIRST AID KIT
FIRST AID is the help given to someone who is
injured or sick before the victim gets further medical assistance. This
help can be given by any person regardless of his/her knowledge in a
medical profession.Whenever an accident occurs, something must be done
immediately to help and save life of the victim. You must always be
ready to give a hand to a victim whenever an accident occurs close to
you. To give aid effectively and successfully, one must have elementary
knowledge on how to assist different victims. If you do not know how to
help a certain victim, you can ask someone to assist instead. Do not
engage yourself in assisting
if you actually do not know where to
start. You may find yourself worsening the situation of the victim
unknowingly. However, this should not be taken as an excuse for failing
to help. Always be ready to render some kind of help.
First aid helps to:
- relieve pain and bring hope to the victim.
- prevent permanent disability
- prevent the victim’s condition from getting worse
- reduce the possibility of death.
- shorten recovery time
CAUSES OF ACCIDENTS IN A CHEMISTRY LABORATORY
Accidents may occur in a school laboratory if utmost care is not taken into account. Accidents in the laboratory are mainly cuts on parts of the body such as hands, fingers, legs or head. Others are burns from flames, scalds from boiling fluids, bruises and grazes due to accidental falling on a slippery floor.
i. Some possible causes of accidents in the laboratory include:Failure to follow the correct experimental procedures for example, pouring water into an acid instead of pouring an acid into water as the rule is.
ii. Neglecting some laboratories rules such as ignoring to wear protective gears, tasting the chemicals, eating or drinking while in the laboratory, etc.
iii. Failure to adhere to proper conduct in the laboratory like running unnecessarily and conducting experiments without your teacher’s or
technician’s permission and guidance.
iv. Improper use or handling of laboratory equipment and apparatus when conducting experiments, which could lead to breakage and in turn cause cuts, bruises, grazes, etc.
v. A slippery laboratory floor which can cause fractures, cuts, bruises, grazes, etc
vi. Accidental spillage of chemicals on body parts such as hands, face, eyes, etc, could lead to burns and damage.
vii. Poor ventilation in the laboratory may cause suffocation (due to inadequate oxygen supply) and poisoning (by inhaling poisonous gases produced when experimenting).
viii. Improper disposal of chemical wastes may result in explosions, burns or even fires.
ix. The leaking of gases from taps or cylinders may cause fires or even explosions.
x. Use of wrong reagents due to incorrect labeling of chemicals or use of reagents or chemicals that have expired may cause burns, poisoning or damage to apparatus or equipment.
xi. Inadequate prior information or knowledge on procedures and hazards associated with certain practical activities or reactants may result in burns, poisoning or explosions.
xii. Loose or improperly plugged electrical appliances may cause electric shock, especially when touched with wet hands and during fixing of sockets.
In general, it can be concluded that most laboratory accidents are a result of negligence and carelessness of experimenters. It is also due to failure to follow the laboratory rules and general safety measures.
FIRST AID KIT is a box in which first aid chemicals, tools and instruments are kept. In the laboratory, the box is usually kept in a
place
where it can be easily reached in case of an accident, preferably on
the wall.Each student must be familiar with the tools and chemicals kept
in the kit
First aid kit and its contents
The table below shows types of chemicals found in a First Aid Kit and their functions.
| Tool/chemical/item | Function |
| First aid manual | Contains guidelines on how to use the items in the first aid kit |
| Sterile gloves | Worn on hands when attending bleeding cuts or wounds to avoid infecting wounds and to prevent direct contact with the victim’s body fluids |
| Sterile dressing | Stops bleeding |
| Antiseptic agent | Cleaning and disinfection of wounds, cuts, bruises, grazes or blisters |
| Soap | Washing hands, wounds and equipment |
| Antibiotic ointment | Prevents infection on cuts and bruises in or near the eye |
| Burn ointment | Applied on burns to prevent infection |
| Petroleum jelly | Soothing broken skin |
| Plaster or adhesive bandage | Covering small wounds or cuts |
| Sterile gauze | Covering wounds to protect them from dirt or germs |
| Eye wash solution | Flushing the eyes or as a general decontaminant |
| Thermometer | Recording body temperature |
| Antibiotic towelettes or cotton wool | Cleaning and drying cuts and wounds |
| Iodine tincture | Dressing fresh cuts and bruises |
| Pain relieving drugs such as aspirin, paracetamol, panadol, etc | Relieving mild pains |
| Liniment | Reducing muscle pain |
| Mild antibiotics | Treating mild bacterial infections on the skin, ear, nose and mouth |
| Gentian violet solution | Applied on minor wounds and treatment of serious heat wounds |
| Hydrogen peroxide solution | Cleaning wounds |
| Methylated spirit (70% alcohol) | Cleaning cuts and bruises |
| Bandages | Dressing wounds and cuts, and immobilizing injured limbs |
| Scissors or razor blade | Cutting dressing materials |
| Dental kit | Treatment of broken teeth, loss of crown or filling |
| Safety pins (small and big) | Splinter removal and securing triangular bandage slings |
| Tweezers | Splinter or stinger removal |
| Resealable oven bag | Container for contaminated articles |
| Moleskin | Applied to blisters or hot spots |
| Triangular bandage | Used as a sling, towel or tourniquet |
| Boiled, clean water | Washing hands and drinking |
| Nasal spray decongestant | Nasal congestion from colds or allergies |
| Torch | Source of light |
| Whistle | Blown to call for help |
The Items in a First Aid kit to Provide First Aid to an Accident Victim
First aid procedures
Sometimes accidents may occur in the laboratory due to some reasons
or the other. Whenever an accident occurs, one must be ready and
prepared to assist.
The following are some of the health problems that may require first aid and the procedure to follow when providing help.
Bleeding
is the loss of blood from the body and
usually occurs from a visible wound. Bleeding may be external or
internal. It may from artery, vein or capillary. Bleeding may be severe
or light. Excessive loss of blood may cause death.
(a) Internal bleeding Signs and symptoms of internal bleeding include the following:
- Bruised, swollen, tender or rigid abdomen
- Bruises on chest, neck, legs or signs of fractured ribs
- Vomiting or coughing up blood.
- Wounds that have penetrated the skull, chest or abdomen
- Bleeding from body cavities such as the ears, nose, rectum or vagina
- Abdominal pulse and difficulty breathing
- Cool, moist skin
- Fractures
Procedure
- First aid in the field for internal bleeding is limited. If the
injury appears to be a simple bruise, apply cold packs to slow down the
bleeding, relieve pain and reduce swelling. - If you suspect more severe internal bleeding, carefully monitor the patient. Be prepared to administer CPR if required (and you are trained to do so).
- Seek medical advice immediately if the situation seems to be worse.
(b) Severe bleeding
Procedure
- Severe bleeding with blood oozing out rapidly must be stopped at once. This can be done by applying direct pressure to the wound. Use a dressing if available. If it is not available, use a rag, towel, piece of clothing or your fingers alone. If the wound is large, press the edges of the wound together but firmly. However, this should be done only if there is no fracture.
- Lay the victim down in a comfortable position.
- If
the wound is on a limb, and provided it is not fractured, raise the
wound above the level of the heart. Then continue to apply direct
pressure. This should be done only if bleeding continues and if it does not cause pain. - If bleeding still cannot be controlled, the next step is to apply pressure at a pressure point. For wounds of the arms or hands, pressure points are located on the inside of the wrist (radial artery-where a pulse is checked) or on the inside of the upper arm (brachial artery). For wounds of the legs, the pressure point is at the crease in the groin (femoral artery).
- When bleeding stops, clean the wound carefully and thoroughly with a suitable disinfectant. Do not remove any objects stuck in the wound, as this would lead to more bleeding.
- Place sterile gauze on the wound and press it down firmly. Cover it with a soft material and hold it in position using a firm bandage. After the bandage is in place, it is important to check the pulse to make sure blood circulation is not interrupted. A slow pulse rate, or bluish fingertips or toes signal a bandage may be hindering blood circulation.
- Seek medical help immediately.
Important:
Once pressure is applied, keep it in place. If dressings become soaked
with blood, apply new dressings over the old dressings. The less a
bleeding wound is disturbed, the easier it will be to stop the bleeding.
(c) Light bleeding
Procedure
- Place the victim in a comfortable resting position.
- Elevate the injured part while applying pressure. This should be done only if the wound is on a limb and you do not suspect a fracture.
- Gently and thoroughly clean the wound using water and antiseptic or common salt solution.
- Cover the wound with sterile gauze or clean dressing dipped in iodine solution.
- Dress and bandage the wound
- Take the victim to hospital if bleeding still continues.
(d) Nose bleeding
Bleeding usually occurs near the tip of the nose. The bleeding may be
a result of high blood pressure, rheumatic fever, or injury. Nose
bleeding is also likely to occur at high altitude because of low
atmospheric
pressure or extreme coldness.

Procedure
- Let the victim sit calmly. This makes the heartbeat to slow down and hence reduce bleeding.
- Loosen clothing around the neck and chest.
- Let the victim sit upright and lean the head forward slightly. By remaining upright, the victim reduces the blood pressure in the veins of his or her nose. This discourages further bleeding. Leaning forward will help the victim avoid swallowing blood, which can irritate his or her stomach.
- Have the victim pinch his or her nose, to keep nostrils shut, using thumb and index finger. Ask the victim to breath through the mouth. Let him or her continue pinching for a few minutes.
- Apply cold, wet compression over the nose, face and at the back of the victim’s neck.
- When bleeding stops, gently clean the nostrils.
- If bleeding does not stop after 20 minutes, take the victim to the hospital immediately.
- To prevent re-bleeding after bleeding has stopped:
- Ask the victim not to pick or blow the nose and not to bend down until several hours after bleeding.
- Let the victim keep his/her head higher than the level of his/her heart.
- If re-bleeding occurs:
- Ask the victim to blow out forcefully to clear the nose of blood clots. Spray both sides of the nose with decongestant nasal spray.
- Pinch the nose as described above and seek medical help.
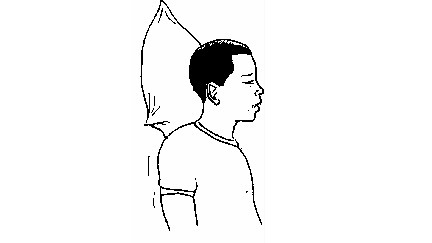
nose-bleeding victim with his head leaned forward.
Suffocation
Suffocation is a condition in which the lungs are not getting enough oxygen, causing difficulty in breathing. In such cases, foams can also appear in the mouth and nostrils. If suffocation is complete (no air at all reaches the lungs), the lack of oxygen and excess carbon dioxide in the blood will cause immediate loss of consciousness. Though the heart continues to beat briefly, death will follow in a matter of minutes unless emergency measures are taken to get breathing started.Suffocation can be caused by drowning, electric shock, gas or smoke poisoning, choking, asthma, severe infections of the throat or other causes.
Procedure
- Remove the cause of suffocation or remove the victim from the cause of suffocation. Loosen tight clothing around the neck.
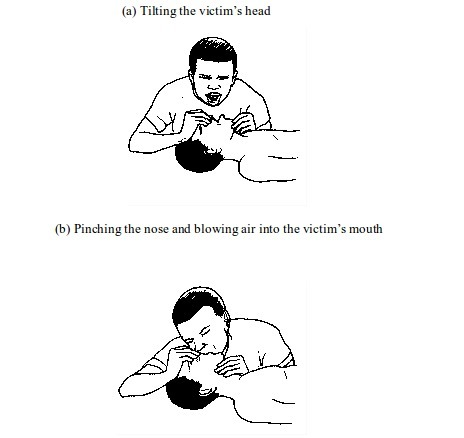
- Make
sure the victim’s airway is open for air to reach the lungs. This can
be achieved by laying the victim on his or her back. Then, with one hand
on the victim’s forehead and the other on the chin, tilt the head
backwards, to open the airway. Tilt the head until the chin points
straight upwards. If the airway is blocked by fluid or solid, remove it. - Administer cardiopulmonary resuscitation (CPR).
This involves blowing air into the victim’s mouth (mouth-to-mouth
breathing). To do this, pinch the nose and close up the mouth. Take a
deep breath and then blow hard into the victim’s mouth. Watch the rise
in chest and repeat the procedure until the victim’s breathing is restored. In case the person does not respond, go for chest
compressions. - Compressions can be done by placing the palm of one hand in the space between the nipples and the other hand over it and
pushing the chest by using your upper body weight. Care should be taken to prevent chest injury or fracture. Two compressions can be given every second or hundred per minute. After thirty compressions, go for mouth breathing again. Repeat the procedure until natural breathing is restored. - Keep the casualty warm using a light blanket.
- Take the casualty to the hospital immediately.
Choking Choking occurs when food or a foreign object blocks the upper part of the windpipe. This interferes with normal breathing. The signs of this problem include difficulty in breathing and speaking. Have you ever been with a person who is chocking? Did you know what to do?When attending a person who is chocking, first notice whether the person can talk, breathe or cough.
Caution: Do not try to slap the person on the back. The slapping may only cause the food to become more deeply lodged in the airway.
Procedure
- Ask the victim to cough up the object.
- If the object remains stuck, give firm but gentle taps between the shoulder plates.
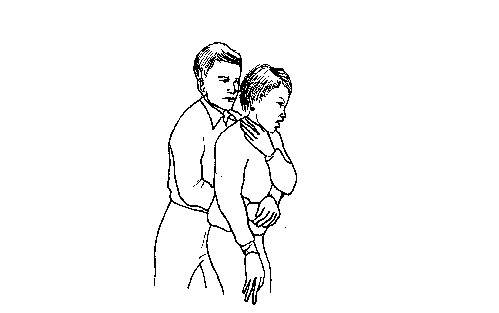
- If the object is still stuck, apply quick abdominal thrusts i.e. Heimlich manoeuvre as follows:
- Stand behind the victim and make him or her lean forward slightly.
- Put
your arms around the person, placing your fist just below the
breastbone. Grasp your fist with the other hand near the top of the
victim’s stomach. - Press your fist onto the victim’s abdomen. Give a series of quick, sharp upward and inward thrusts to dislodge the object.
Poisoning
A poison is any substance that can harm the body or seriously
endanger health when taken into the body. A poison may get into the body
through inhaling, swallowing, wounds or skin cuts or absorbed into the
body in other ways. Potential poisons include poisonous fumes (or
gases), laboratory chemicals or even medical drugs and medicines.In
every household, there are different kinds of things that are poisonous.
Some are deadly even on a very small dose. Others may be more
or less harmless when taken in small quantities.Examples of poisonous substances at home are kerosene, disinfectants, paints, medicines, artificial fertilizers, etc.
Some signs and symptoms of poisoning are nausea, vomiting, abdominal cramps, pain, difficulty breathing, diarrhea, and abnormal skin colour. Others are breath that smells like the chemicals, chemical burns, empty medication bottles or scattered pills, sleepiness, confusion or other unexpected signs.
Procedure
- Call for medical help immediately if the person shows one or some of the following signs: drowsy or unconscious; having difficulty breathing or has stopped breathing; uncontrollably restless or agitated.
- For the time being, find out what caused the poisoning, i.e. look for the poison and identify it.
- If the person has been exposed to poisonous fumes such as carbon monoxide, get him or her to where there is fresh air immediately.
- If the poison is in the eye:
- Wash the eye with a lot of clean water.
- Ask the victim to blink as much as possible.
- Do not rub the eye.
- If the person swallowed the poison:
- Remove anything remaining in the mouth
- Induce
vomiting if the poison is not strong acid or alkali as these are
corrosive substances. Non- corrosive substances include medicines and
soaps. Vomiting can be induced by inserting your finger in the victim’s throat until the finger touches the epiglottis. - Do not induce vomiting if the poison swallowed is corrosive. Corrosive substances include kerosene, bleach, detergent, laboratory acid, disinfectant or certain toiletries. If the suspected poison is a household cleaner or other chemical, read the label and follow instructions for handling accidental poisoning.
- Neutralize the poison by giving the victim plenty of milk to drink, an egg white or water.
- If the poison is on the skin:
- Remove any clothing from the affected part.
- Wash the affected area thoroughly with a lot of water.
- Do not apply any ointment.
- Make sure the person is breathing. If not, start rescue breathing and CPR.
Note: Take the poison container or any pills, pill bottle or pill blister pack with you to the hospital.ShockShock
is a condition in which the body system fails to take enough blood to
the vital organs. If untreated, it can lead to permanent organ damage or
death. The vital organs include the heart, the lungs, the brain, the
kidneys and the liver.
Causes
Shock may result from bad news, heatstroke, severe illness, blood loss,
dehydration, poisoning, severe burns, an accident or other causes.
Signs and symptoms
Various signs and symptoms appear in a person experiencing shock. They include the following:
- The skin becomes cool and moist. The skin, lips and fingernails may appear pale or grey.
- The pulse rate is weak and rapid. Breathing may be slow and shallow.
- The limbs may tremble and get weak.
- The eyes lack lustre and may seem to stare. Sometimes the pupils are dilated.
- As
the shock develops, the victim may experience nausea and even vomiting.
Eventually he or she may become restless, nervous, aggressive and
finally conscious or unconscious. If conscious, the person may feel
faint or be very weak or confused.
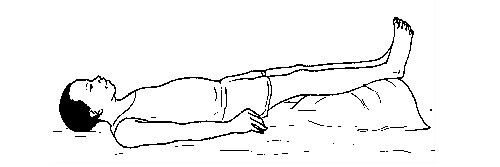
Procedure
- Control any cause of shock such as bleeding, etc.
- Lay the person down with his or her feet higher than his or her head (shock position).
- Loosen tight clothing, laces, belts and shoes.
- Turn the person on his or her side to prevent choking if the person vomits or bleeds from the mouth.
- Keep the person warm and comfortable if he/she feels cold. Cover the victim with a blanket or any heavy clothing.
- Seek treatment for injuries such as bleeding or broken bones.
- Administer CPR if the person does not appear to breath well, cough or even move.
- Seek medical help immediately.
The shock position
Electric shock
An electric shock occurs when a person comes into direct contact with
electricity. Exposure to electricity may result in injury or even
death.
Injuries may be burns, or physical injuries that result from being thrown by the electric current.
Procedure
- Switch off the main switch immediately if possible.
- If
not possible to put off the switch, detach the casualty from the source
of electricity using a non-conducting object such as a dry wooden
stick, cardboard, plastic or rope. - Loosen any tight clothing, necklaces, bangles, etc.
- If the person is unconscious, apply mouth-to-mouth respiration (CPR) immediately.
- Treat for shock, burns, bruises or other injuries the victim may have sustained. Lay the victim down, and if possible, position the head slightly lower than the trunk, with the legs elevated (shock position).
- Take the person to the hospital immediately.
Caution
- Do not touch the person with your bare hands if he or she is still in contact with the electric current.
- Do not get near high-voltage electricity until power is turned off. Instead, call for help immediately.
- Do not move a person with an electrical injury unless the person is in immediate danger.
Bruises
A bruise is an injury beneath the skin. Bruises can be identified by pain, swelling or a mark under the skin. A bruise forms when a blow (hard hit) breaks the blood vessels near the skin’s surface. This allows a small amount of blood to leak into the tissues under the skin. The trapped blood appears as a blue-black mark.Procedure
- Wash the bruised part of the body.
- Apply a cold compress, such as a cloth dipped in cold water or ice wrapped in a cloth, to the injury. This helps reduce pain, swelling, and speeds up recovery.
- If the bruise is on a limb such as arm or leg and it covers a large area, keep the limb elevated as much as possible for the
past 24 hours. - After 48 hours, apply a cloth dipped in tepid water to the bruise for about 10 minutes, three times a day. This will
help increase blood flow to the affected area and thus speed up healing.
Vomiting
Vomiting is an involuntary ejection of the contents of the stomach through the mouth.
Possible causes of vomiting
- Allergic reactions
- Diseases e.g. malaria
- Physiological condition e.g. pregnancy
- Food poisoning
- Unpleasant smell or taste
- Drinking contaminated water.
- Inhaling poisonous fumes
- Over-eating.
Procedure
- Give the victim an oral rehydration drink or oral rehydration salt solution. You may also provide a lot of any clear fluids.
- Allow the person to have a complete rest.
- Take the victim to hospital if:
- Vomiting continues persistently.
- The victim vomits blood.
- The victim experiences high fever.
- The victim is very dehydrated. This will be observed when the mouth and skin become very dry.

Fainting
Fainting is a sudden loss of consciousness caused by a temporary fall
in the supply of blood and oxygen to the brain. Sometimes it can be
caused by emotional shock or prolonged standing. The person feels weak,
sweats and
then falls down.
Procedure
- Loosen or remove any tight clothing from the victim.
- Make the victim lie down on his or her back.
- Raise the legs of the victim (shock position) above the level of his or her head. This will increase the flow of blood to the brain.
- Make sure the victim is exposed to plentiful supply of fresh air.
- If there is no improvement in a few minutes, rush the victim to the hospital
Caution:
Do not try to warm the victim Muscle cramps
A muscle cramp is an involuntarily and forcibly contracted muscle that does not relax. A muscle that contracts involuntarily is called a ‘’spasm.’’ If the spasm is forceful, it becomes a cramp. Therefore, muscle cramps occur because of uncontrolled muscle spasms.
Signs and symptoms
- A sharp, sudden and painful spasm, or tightening of a muscle (especially common in the legs).
- Muscle hardness
- Twitching of the muscle
- Persistent cramping pains in the lower abdominal muscles.
Causes
- Imbalance in certain minerals, body fluids, hormones, and chemicals that allow stretching and contracting of our muscles.
- Malfunctioning in the nervous system.
- Excessive physical activity and hormonal imbalances cause us to sweat. This brings about the loss of many essential minerals, such as potassium and calcium, which our body muscles need.
Procedure
- Lay the victim down
- Gently massage and stretch out the cramped muscle(s).
- Apply some anti-cramp ointment to the affected area
- If the problem persists, seek medical help immediately.
Hiccups
Hiccups are caused by sudden involuntary contraction of the diaphragm
muscles, giving a characteristic ‘’hic’’ sound. Numerous cures for
hiccups exist.
These cures are thought to work because they increase
the level of carbon dioxide in the blood, which usually stops hiccups.
(See procedure 1-3 below). If the vagus nerve that runs from the brain
to the stomach is stimulated, hiccups can also be alleviated.
Procedure
- Give the affected person a polythene bag and encourage him/her to re-breath her own expelled air.
- Ask the person to drink a glass of cold water quickly.
- Tell the victim to hold his/her breath for as long as possible.
- Ask the victim to pull on his/her tongue.
- The victim may swallow finely crushed ice.
- Children can be given a teaspoonful of a weak solution of sodium bicarbonate or lemon juice.
- Place one teaspoonful of dry sugar or honey on the back of the victim’s tongue. (You can repeat this process 3 times at 2-minute intervals)
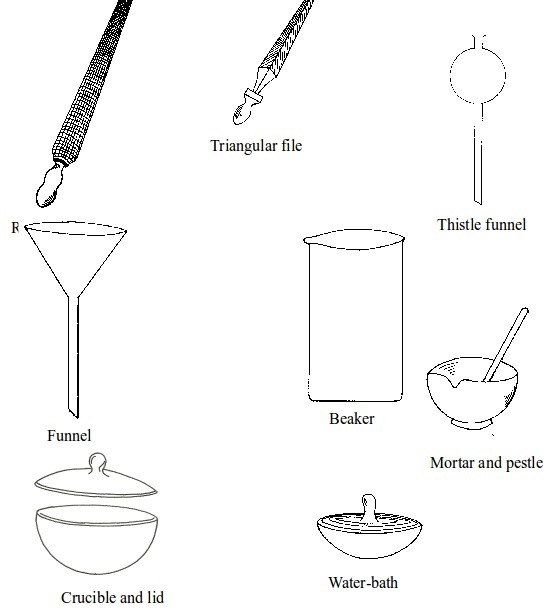
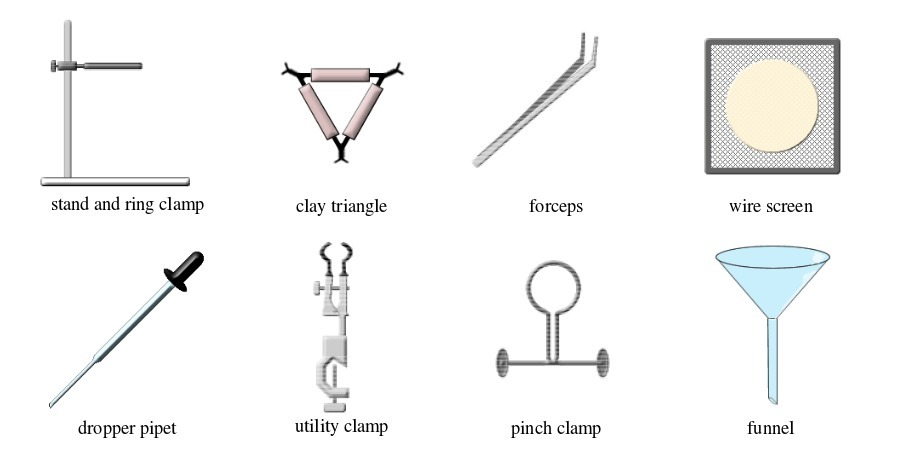
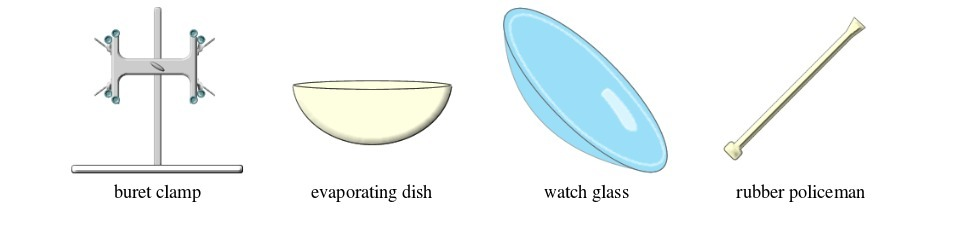
BASIC CHEMISTRY LABORATORY APPARATUS AND THEIR USES
Instruments used for carrying out different experiments in the laboratory are called laboratory apparatus. Laboratory apparatus can be classified according to their uses as:
- apparatus for holding things e.g. test-tube holder, retort stand and clamp, test-tube rack, tongs and tweezers;
- apparatus for taking measurements e.g. thermometer, burette, pipette, measuring cylinder, measuring flask, beam balance, electronic balance, common balance, measuring syringe, beaker and stop watch;
- apparatus for heating substances e.g. boiling tube, pipeclay triangle, crucible and lid, wire gauze, deflagrating (combustion) spoon, Bunsen burner, spirit lamp, tripod stand, evaporating dish, wire gauze and stove;
- apparatus for doing chemical reactions (or testing) e.g. beaker, test tube, dropper, flask, watch glass, gas jar and thistle funnel;
- apparatus for filtering e.g. filter funnel, filter paper and cotton wool;
- apparatus for grinding e.g. mortar and pestle;
- apparatus for storage e.g. reagent bottles and wash bottle;
- apparatus for scooping e.g. spatula; and
- apparatus for safety e.g. goggles and hand gloves.
The Apparatus Used in a Chemistry Laboratory
Some chemistry laboratory apparatus
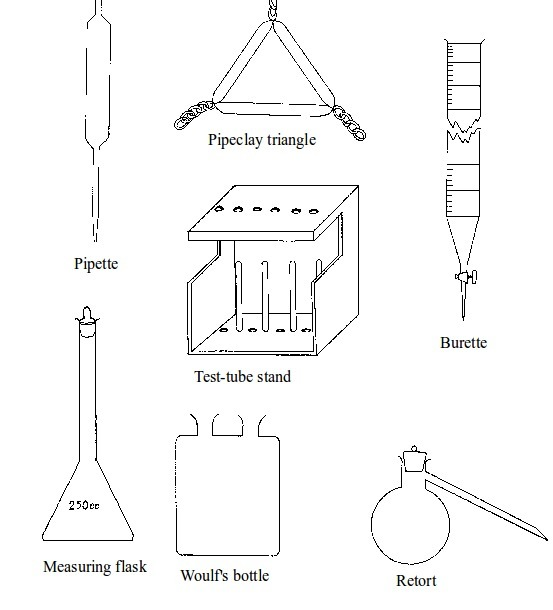
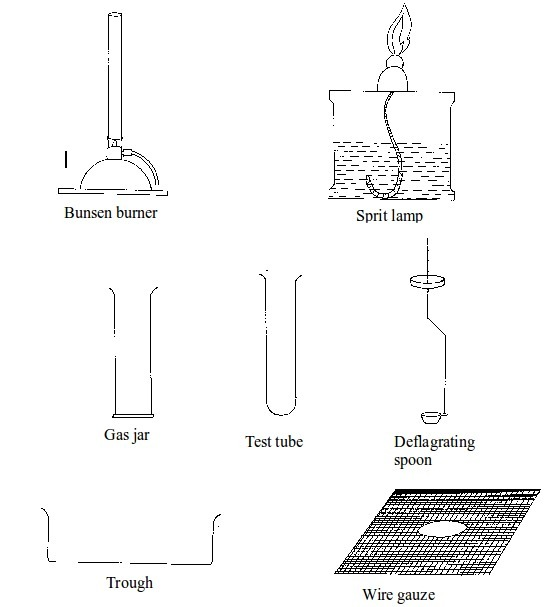
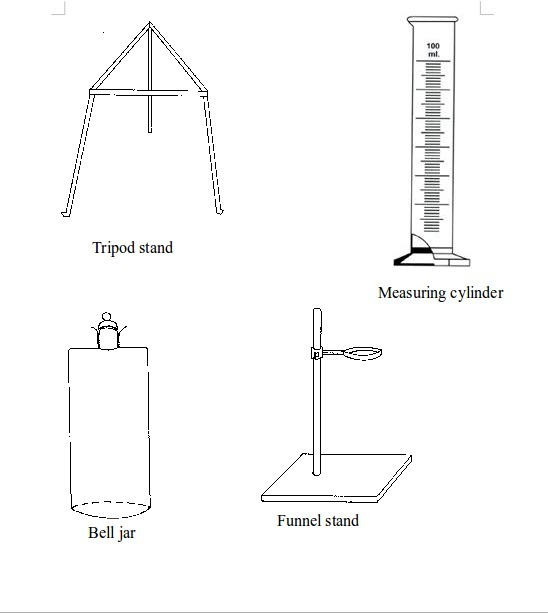
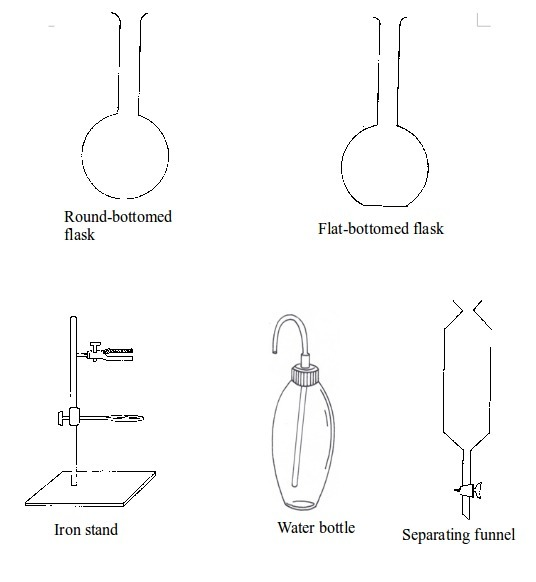
Chemistry Laboratory Apparatus According to their Uses
| Apparatus | Material | Uses | |
| 1. | Test tube | Glass | Holding chemicals or, heating substances |
| 2. | Funnel | Glass or plastic | Leading liquids into containers, and for filtration purposes |
| 3. | Beaker | Glass or plastic | Holding, heating, and mixing liquids |
| 4. | Flask | Glass | Holding, heating, and titrations |
| 5. | Retort stand | Metal (iron) | Holding apparatus during heating |
| 6. | Tripod stand | Metal (iron) | Holding apparatus during experiments |
| 7. | Gas jar | Glass | Gas collection |
| 8. | Wash bottle | Plastic | Washing |
| 9 | Crucible | Ceramic or non-reactive metal | Heating |
| 10 | Test tube holder | Metal and plastic or wood | Holding test tubes while heating |
| 11. | Weighing balance | Metal and plastic | Measuring weight (or mass) |
| 12. | Spatula | Metal | Scooping small quantities of powder or crystalline chemicals |
| 13. | Condenser | Glass | Cooling hot liquids |
| 14. | Pipette | Glass | Accurate measurement of specific volumes of liquids for titrations |
| 15. | Burette | Glass | Titrations |
| 16. | Trough | Glass | Assists in gas collection |
| 17. | Tongs | Metal | Picking and holding hot substances and apparatus |
| 18. | Measuring jar | Glass | Measuring volumes of liquids |
| 19. | Thistle funnel | Glass | Leading liquids into containers and apparatus |
| 20. | Dropper | Glass and rubber | Dropping indicators into reagents |
| 21. | Mortar and pestle | Clay | Crushing or grinding substances |
| 22. | Wire gauze | Metal | Even distribution of heat during heating |
| 23. | Spring balance | Metal | Measuring weight |
| 24. | Distillation flask | Glass | Distillation |
| 25. | Combustion spoon | Metal | Burning powder in jars |
| 26. | Thermometer | Glass and liquid metal | Measuring temperature |
| 27. | Delivery tube | Glass | Allowing gases pass through |
| 28. | Bunsen burner | Metal | Heating substances |
| 29. | Separating funnel | Glass | Separation of immiscible liquid mixtures |
| 30. | Measuring cylinder | Glass or plastic | Measuring volumes of liquids |
| 31. | Measuring syringe | Plastic | Sucking in and measuring specific volumes of liquids |
| 32. | Stopwatch | Plastic or glass and metal | Accurate measurement of time |
| 33. | Watch glass | Glass | Used as a surface to evaporate some liquids, to hold substances being weighed or observed, or as a cover for a beaker |
| 34. | Boiling tube | Glass | Is a large test tube used to heat substances requiring strong heating, or when the sample is too large for a test tube |
| 35. | Evaporating dish | Ceramic | Heating and evaporating liquids and solutions |
| 36. | Filter paper | Paper | Filtration |
| 37. | Test tube rack | Wood or plastic | Placing test tubes |
| 38. | Reagent bottle | Glass | Storing different chemicals |
| 39. | Wash bottle | Plastic | Storing distilled water |
| 40. | Safety goggles | Glass | Protecting eyes from chemical spills, strong light and harmful vapours |
| 41. | Bell jar | Glass | Keeping gases, moisture, air, etc. or creating vacuums |
Common Chemistry Laboratory Apparatus

Activity 1Your teacher will guide you how to measure the volume of liquids using the other apparatuses.
Aim: Tomeasure volume of liquids using different apparatus
Materials: pipettes, burettes, measuring cylinders, water, beakers.
Procedure
- Pour some water into a graduated measuring cylinder with a capacity of 100 cm3. Add the water, one drop at time, up to a 25-cm3 mark.
- While adding water, position yourself at eye-level with the mark on the cylinder. This will enable you to obtain the most accurate measurement. To simplify the work of reading the level of the water, you may use coloured water.
- Select a volumetric flask measuring 50 cm3. Pour the water into the flask until it reaches the mark on the flask’s neck.
- Position yourself at eye-level with the mark. You will obtain the most accurate reading when the mark appears straight rather than elliptical. To obtain this, put a flask on a flat table.
- Add water one drop at a time. Do so until the bottom of the curved surface of the water exactly matches the mark on the flask.
Activity 1.2
Aim:To measure the masses of solid substances
Materials: chemical, electronic or spring balance, watch glasses, various substances such as sand, sugar, salt, flour, stones, fruits.
Procedure
- Put an empty watch glass on the weighing balance. Note down its mass. Record this as mass M1.
- Place the various items you have on the watch glass, one item at a time. Note down the mass. Record this as M2.
Note: to obtain the mass of an object, we subtract the mass of an empty watch glass from the mass of the watch glass and the substance. That is, M2 – M1. For example
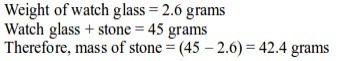
WARNING SIGNS
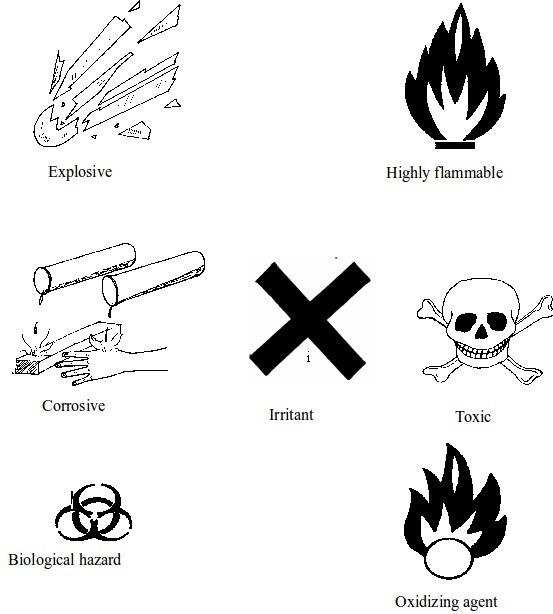
Chemical warning signs are safety symbols found on containers, especially those used in the laboratory. The symbols are also found on tanks or containers that are used to carry, store or transport certain chemicals. Containers holding flammable fuels such diesel, petrol and natural gas, as well as those containing toxic chemicals normally bear warning symbols. These symbols indicate the danger (hazard) likely to be caused by the chemicals they contain if carelessly handled.When performing experiments in the laboratory it is important to read the safety signs on chemical containers. This will minimize the chances of causing accidents in the laboratory.
The Basic Chemical Warning Signs
The Concept of Warning Signs
Before conducting any experiment in the laboratory you must be aware whether the chemical you want to use is toxic, corrosive, flammable, oxidant, explosive or harmful. This information will help you know how to handle the chemicals safely. Proper handling of chemicals enables you avoid unnecessary accidents.
Below is an explanation pertaining to some hazard labels represented by the symbols above.
Toxic, substances include those that can poison you
or the other person working close to you in the laboratory. These
substances can kill within a short time or after some few days. They
should not be allowed to get into your body through body orifices
(month, nose, eyes, ears, etc).
Neither should they be allowed to
contact your skin. They become even more dangerous when they get into
the body. If it happens that these substances touch your skin
accidentally, wash it immediately with ample water.
Corrosive, substances refer to those chemicals that can burn or corrode (eat away) your skin. They can also corrode wood or metals. One can become blind if such substances accidentally get into his/her eyes. If they contact your skin, wash it immediately with a lot of water. Examples of corrosive substances commonly found in a school laboratory are concentrated mineral acids such as sulphuric acid, hydrochloric acid and nitric acid, and concentrated alkalis such as sodium hydroxide, potassium hydroxide and ammonia.
Flammable, These chemicals catch fire easily. For this case, they should be kept away from flames or fires. They can be set into fire by any kind of sparks, be it from welding or fire. When working with flammable chemicals in the laboratory all burners must be put off. These chemicals are usually very volatile. The containers used to carry them must be stoppered immediately after every use. Examples of flammable chemicals are methylated spirit, ether, acetone and methanol.
Explosive, chemicals are those that explode rapidly
upon detonation (set into fire or ignited). Because the reaction is
rapid, it results into throwing off particles at a high speed. For this
reason, they should not be kept in glass containers. This is because
during explosion the particles will disperse around and cause serious
injuries to people. Those explosive chemicals that can react without
external detonation are even more
dangerous
Oxidizing agents, These chemicals can stimulate a burning substance to burn efficiently and
faster.
Therefore, they must be kept away from fires no matter how small that
fire may be. An example of oxidizing agent is oxygen gas.
Harmful or irritant, Harmful substances are those that can impair your health or make you fall sick.
They
do not normally kill instantly but have detrimental effects following a
long exposure to them. These chemicals do not kill immediately.
However, care must be taken when handling or dealing with them.
Irritating substances cause pains when in contact with the body.
They are dangerous to health when in contact with the body surface for a long period of time.


No comments:
Post a Comment